This article has been prompted by personal reasons. I have been applying a non-planetary Feynmann adaptation of the planetary Bohr model to molecular structure (not dealt with here). I thought I should learn a little bit more about the historical antecedents/compulsions that drove Bohr to his model. The second reason is that being a Bengali (sometimes very proudly) 1913 is also the year that Rabindranath Tagore got his Nobel prize for Bengali literature! The first reason will form the main part of the blog.
Two 1913 Nobels
One sees virtues in the Bohr model in modern times,
mainly because of its historical impact. As late as last year (Feb 2012),
Jennifer Oulette, (who maintains a blog called Cocktail Party Physics in the
Scientific American) defended the Bohr model in her article “Don’t Be Dissin’
the Bohr Model!” because of its use as a tool to introduce the
quantum model for the atoms:-
Thomson’s so-called plum pudding model has
incorporated in it the idea that an atom’s positive
mass is spread out diffusely. Rutherford was fortunate to have skilled
assistants such as Geiger and Marsden who set up all the instrumentation for
studying alpha-particle scattering. Indeed, it seems, it was Marsden’s first
attempts that led to the observation that a few of the alpha particles did not pass through a gold foil (as expected)
without changing angles (without scattering) but a few of them bounced back in
an opposite direction as if they had struck a solid wall!
Rutherford’s 1911 paper spent considerable time on the experiments of Crowther with b rays that seemed to support Thomson;s model. In private correspondence with Bragg, Rutherford writes (fom Mansoor Niaz’s article, From Cathodes Rays to Alpha Particles to Quantum of Action...) “...I have looked into Crowther’s scattering paper carefully, and the more I examine it the more I marvel at the way he made it fit (or thought he made it fit) J. J.’s theory . . . I am quite sure the numbers of the earlier part of the curve [Crowther’s] were fudged.”
Two 1913 Nobels
In July of 1913 Niels Bohr’s paper “On
the Constitution of Atoms and Molecules” was published in the first twentyfive
pages of Volume xxvi of the Philosophical magazine. This publication changed
the way we look at small things --- the quantum theory for atoms --- when it
was thought rhat “ … the
laws of classical physics should be universally valid, even at the atomic level”.
That laws of classical physics should prevail at all levels seemed to be a
perfectly valid assumption considering that much of planetary physics,
cosmology, particle physics continue to benefit from the laws of classical
physics that were enunciated before the twentieth century.
In the same year Rabindranath Tagore
was given the Nobel Prize for Literature ( ... the first
non-European to win the prize) a matter
of great intellectual pride for many Indian intellects who probably now felt at
par with the European. Tagore himself (according to Wikepedia) descended from a
shunned family of Brahmins because some of them were converted to Islam under
the ruler Pir Ali (hence Pirali Brahmins). Tagore may have been re-embraced by
the Brahmin community as an European although he was only penning folk thoughts
inherited over tens of millennia. We will certainly celebrate Tagore’s Nobel centenary
with more zeal than we will celebrate the Bohr model. We claim to have
lost Tagore's Nobel medal, although we see o be sure it is likely to be tucked away carefully in some locker
of a very less noble collector.
The Bohr’s model and Tagore’s Poems seemingly reflects
a triumph of left- (analytic) and right- (synthetic) brain activities. two mutually exclusive attitudes, somethimes.
Tagore’s poems reflect ancient native thoughts that come from our
experience/acceptance of the four elements. Bohr’s mind-function is perhaps
more common among Europeans than it is among colonized Indians who have been
trained by Europeans to carry out their instructions.
This contradiction is somewhat galling for us. In
the western mind we Indians are appreciated for the thoughts of our right-brain
past. Our left-brain activity in the present is rarely a matter of much
competitive concern no matter how much we rave and rant about our
post-independence II institutions (IITs and IISERs and IIMs). Quite simply put,
we are not innovative enough (emphasis on enough).
When
invited to speak at S’eminaire Poincaré in
2007, the young (by my age standards) Jûrg Frôhlich made this opening remark:-
The 21st Century appears to witness a fairly strong decline in Society’s
– the public’s, the politicians’, the media’s and the younger generations’ –
interest in the hard sciences, including Physics, and, in particular, in
fundamental theoretical science based on precise mathematical reasoning. It is
hard to imagine that reports on a discovery like the deflection of light in the
gravitational field of the sun and on the underlying theory, general relativity, along with a
photograph of its creator, Albert
Einstein, would make it onto the front pages of major daily newspapers,
as it did in 1919
I couldn’t agree with him more. The young have
their multimedia and their face-book/twitter/multi-media buzz to worry about.
They have our mistakes to repeat. We cannot grudge them that, This blog is
(hopefully) to put less trivium in trivial pursuits.
This indifference to matters scientific or even matters that are seen
remotely intellectual pervades the Indian mind-set, including even that of our
intellectual community (in a way). Part of our problem, is that we are not
observative, inquisitive, nor creative – we are always derivative. (= copied,
unoriginal, imitative, plagiaristic, lacking in originality; Thesaurus) without
being integrative.
I can’t deduce why. I presume living is easy in this land of birds and
bees and bovines; sustaining a reasonably enjoyable life is not a problem. When
defending that life we are happy to be casted and scheduled and ruled. We have not really learnt to mark out an
intellectual kingdom, build upon it, preach, defend, impose and spread the
kingdom and reap its benefits thereby.
I have not taught enough (emphasis on taught).
It’s not the learning one needs to worry about. It’s about being innovative
that one needs to worry our right-brain-activities about.
The idea in this blog, marking Bohr’s hundred, is mainly to record the atmosphere that led to way Bohr’s “old” quantum mechanics. I
don’t expect that this topic will attract more readers than I usually do. At my
age I don’t understand the indifference of the multi-mediated young minds to matters
which I think are vitally important for a social outlook that comes from
erudition and not from hip-hop-hype.
As a nation, we have the problem that we tend to think that all
that is to be known is already mentioned in our scriptures even without a Hindutva saffron colour of the
government at the centre. I happened to read a 1918 Nature review on Hindu Achievement in Exact Science by a Benoy Kumar Sarkar. The review writes “... Apparently the author thinks that mere mention of a subject in ancient history proves that the person who has mentioned it discovered all about it. ... a considerable deficiency of the power of accurate scientific judgment, ... requires
practical evidence in the first place....” I will tend to agree with this assessment. There is life beyond vedic knowledge especially when we are not those who claim to have the same colour as the top of our Indian flag and, not coincidentally, the same ranking in the Indian community .
In any case, one think that our Vedas do not definitely seem to have is any model for an atom (anu is not an atom... see my blog).
This blog is to just stroll through the efforts that led to Bohr and his
model. This will basically be my interpretation of the way good science is done
without closure. It requires a society waiting for one step of the science to
be climbed before the next step is seen for further progress step by step. We
require a new breeding procedure for science that is not a step (or many steps) behind the frontiers but a new breed of scientists --- a new science gotra --- that creates frontiers.
Pitfalls in Making Models
There are many scientists who are aware of
experimental facts and theories that are said to explain them. For some reason,
usually intuitive and rarely deductive to begin with, the scientist (let us say)
would like to try out another theory ... just for the heck of it, say. So he
(this includes the she) start turning things on their heads in his mind (Like
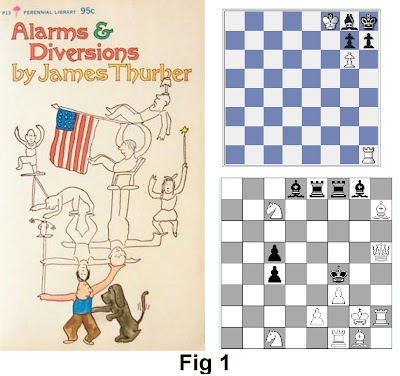
the person in Thurber’s cartoon, Fig 1, left). He has to resort to many other
conjectures and posits available to him to plug loopholes and prevent his,
theoretical model from toppling all over him.
He hope by this complicated model he would eventually crown his feat
with national recognition ... this usually requires positioning another
scientist on the top balancing another hypothesis. He does this until, his pet dog, say his
mathematics, tickles his funny spot. The theory collapses, in good humour, and
the next balancing act starts.
Its like a game of chess where the new scientific model is
white, and black is the defending old model, Black’s stratetegies for defence takes all
possible attacks on the model into account. Black thinks heis well fortified
until he reaches a stage where any further defence move will only expose his
king to a check-mate. This is a zugzwang in
chess. Two problems from the internet of white mating in two moves (Fig 1,
right) illustrates this state. In logic, I guess, zugzwang is the equivalent of reductio
ab absurdum (“reduction to absurdity” is a common form of argument which seeks to
demonstrate that a statement is true by showing that a false, untenable, or
absurd result follows
from its denial; Wikipedia).
Models for Atom without Planck's constant
This blog is not required to inform the reader about the history of the Bohr Model. I have learnt from various articles available on the internet. These include Charles Baily’s Atomic Modeling in the Early 20th Century: 1904 – 1913 (referred to as Baily); Helge Kragh’s Before Bohr: theories of Atomic structure 1850-1913 (Kragh1) and The Early Reception of Bohr’s Atomic Theory (1913-1915)- A Preliminary Investigation (Kragh2) and The gallery of failed atomic models, 1903-1913 (http://skullsinthestars.com/2008/05/27/)
Bohr’s model of the atom is the logo of the Atomic energy commission of the United States of America (Fig 2 left), “The persistence of the ubiquitous symbol reflects its ancestry: Bohr’s atomic theory imitates in part Newton’s planetary theory ....The “... curious blend of traditional and modern ideas that underlies Bohr’ theory has given it a remarkable vitality. The language and spirit of Bohr’s work have persisted in common thought long after the model itself was commonly acknowledged (by Bohr among others) to have served its purpose. “
The logo of Kragh’s department (Fig 2, middle) at Aarhus University,
Denmark, is meant to “combine scientific with the popular” which it does with
an instantly recognizable depiction of what could be a Bohr-like atom asserting
thereby the way the Bohr model is accepted by popular imagination. An
interesting feature of the Aarhus logo is it combines another “popular
imagination” fractal-like five-fold geometry. It reminds one of another popular
five geometry obtained from Penrose-tiling (Fig 2, right). The evolution of
logos follow the evolution in science..
Bohr’s July 1913 contribution saw an immediate (considering
those days when all communication had to be by mail) national recognition in The Times newspaper in September 1913
when they referred “... to Jeans’s praise of the young
Danish physicist and his new theory of the hydrogen spectrum...” The Bohr model is now thought to be terribly passé (out-of-date) because of its
planetary two-dimensional nature, and most importantly, because of its failure
to be applicable to the simplest
molecule --- the hydrogen molecule. We will examine this aspect in the next blog.
One sees virtues in the Bohr model in modern times,
mainly because of its historical impact. As late as last year (Feb 2012),
Jennifer Oulette, (who maintains a blog called Cocktail Party Physics in the
Scientific American) defended the Bohr model in her article “Don’t Be Dissin’
the Bohr Model!” because of its use as a tool to introduce the
quantum model for the atoms:-
There’s ...
shortcomings related to predictions about the spectra of larger atoms and the relative intensities of spectral lines, yadda,
yadda, yadda, but we’re focusing on the most major objections for the sake of
simplicity. ... To understand why physicists discarded the Bohr model, you’ve
got to delve into the mind-bending intricacies of quantum mechanics, and
explain all kinds of things the average person likely has never encountered in
any real depth: wave functions, uncertainty, superposition of states, spectral
lines, and so on. I prefer the Bohr model to introduce non-scientists to the
basics of atomic structure
These comments do not have a gospel truth about them; they
are meant to influence the very influential cocktail party crowd. To have a
column on “Cocktail Party Physics” in the Scientific American is perhaps a sign
of the times considering that there used to be a Martin Gardner (1914-2010)
column --- among others --- on Mathematical Games (see Douglas Hopfstadter’s Martin Gardner: A Major Shaping Force in My
Life, Scientific American, May 24 2010).
It is
important to note that Bohr’s model for the hydrogen atom is really a
first long-lived stationary point of evolving models for the atom. Democritus’
atom as an indivisible aspect of matter which latter day Indian philosophers
may have mistaken as the anu of adristam, (see Parts I and II of my
blogs on God and God Particle) is quite different from the models of atom that
came especially after the Thomson-Rutherford models. In Dalton’s atomic theory, atoms of
different elements had nothing “substantial in common. The first indication
that there is a commonality among the elements was perhaps due to William Prout
around 1815 when he thought that all elements were made up of integral number
of hydrogen atoms. This attempt was rejected primarily because of the
observation of a definitely non-integral number of hydrogen atoms required to
account for the atomic weight of chlorine.
When Bohr
started his work on the structure of atoms there was not much interest in the
physics community in the subject as a do-able problem. “Referring to the
period about 1910, … for the average physicist of the time, speculations about
atomic structure were something like speculations about life on Mars – very
interesting for those who liked this kind of thing, but without much hope of
support from convincing scientific evidence and without much bearing on
scientific thought and development.”(Kragh1).
It is probably the kind of work that will be equivalent in recent times to the
work on teleportation in the context of H. G. Wells’ Time Machine.
Etched in
the history of the Bohr model for the atom must, I suppose, have references to
the men in Fig 3 --- Angström, Balmer, Thomson, Haas, Planck, Rutherford,
Einstein and Bohr.(not necessarily in chronological order) and with some
important exceptions such as Rydberg and Nagaoka (see later). The impact of Planck, Einstein and Bohr will be considered in the ext blog.
If one
wants to go back to it all on the story of Bohr’s model, one must start perhaps
with the prisms in fairs and the dispersion of light in rainbow colours. These
prisms are associated with gypsies and their chandeliers. I think one can say
with all fairness that one (even one of our know-all vedic scholars) does not
know who discovered/invented the prism. One always remembers the elemental thrills
when the dispersion of colour is seen from those simple triangulated glass
objects. Yet, what a story these simple prisms had for science!!!
Fraunhopfer
(early 1800s) is credited with the creation of the spectroscope and quantifying
the solar spectrum as well as the positions of dark lines in the spectrum. The spectral lines were thought to
be in chaotic positions and it was recognised that the solar spectrum had no
heavenly single-god characteristic, Kirchoff and
Bunsen (1850s) could identify these lines with elements. Anders Jonas Ångström was
setting the foundations of spectroscopy from sparks and the sun from which he
proved that the sun’s atmosphere is mainly hydrogen in 1862. He obtained and
tabulated large number of spectral lines the inaccuracy of which was mainly due
to the inaccuracy of the metre scale that he used for measurement. At that time
such lines were of interest to astronomers who spent their time looking at the
sky and worried about motions of stars and planets. It is therefore of little
surprise that the model of the atom should evolve from the science of planets. The
spectral lines of Angstorm (~1868) remained in Tables and charts for about
twenty years. Little systematic was found although several attempts were made
for the next twenty years. In particular, there was some hope of finding some regularity
in the spectral lines of hydrogen.
It was at this time that Johann Balmer found the
famous systematic that is now known as the Balmer formula for what is now known
as the Balmer series of spectral lines in hydrogen. Balmer published this paper
(the first of only two that he wrote) at the age of sixty, the age at which
Angstorm passed away after spending a major part of his scientific life with
spectral lines. Balmer took up mathematics for study and was a school teacher.
He married late at 43 and between 43 and sixty he begat six children. When he
finally found time from family-ing activities, he looked at the spectral lines
of hydrogen and by looking at the position of four hydrogen lines in the
visible spectrum he was able to conjecture up a formula. This formula correctly
“predicted” another line in the near ultraviolet that he did not know existed.
The Balmer rule (1885) for the wavelength, l of the
lines in the spectrum is
lm = Bm2/(m2 – 22)
Where m > 2 is an integer
and B is a constant. Balmer, it seems, did not give the method by which he got
his formula in his first paper. He was able to look at a series of numbers and
get a general formula for the series in a converse manner of Ramanujan who was
able to look a series and get the limiting values.without really requiring proving
it. Balmer could not get the correct positions for the lines of helium.
Rydberg generalized (1888) Balmer’s formula to the with
1/l = RH(1/22
– 1/n2) = (4/B)(1/22 – 1/m2)
where m is an integer with m = 3, 4, 5. This is a special case of
the general expression (1889)
1/l = RH(1/n2 – 1/m2) (m > n = 1, 2, .... m)
The lines for hydrogen has only n = 2. The Lyman series for hydrogen lines
discovered later (1906) has n = 1, the Paschen series (1908) has n = 3,
These three series were known to Bohr. The Brackett series (1922) and
the Pfund series (1925) were n = 4
and n = 5, respectively corresponded
to Rydberg’s formula, This is the formula that was first explained by Bohr.
Planetary Models and Saturn’s Rings
The early models for the atom did not attempt to explain the Balmer
lines. Perhaps, the first planetary model is by Nagaoka who published a version
in 1903 before publishing it in the Philosophical magazine in 1904. Nagaoka’s model
drew heavily from James Clerk Maxwell’s treatment of Saturn’s rings. Maxwell admits that he did this model purely
for the love of it and not for any commercial reason.
But when we contemplate the Rings from a purely scientific point of
view, they become the most remarkable bodies in the heavens, except, perhaps,
those still less useful bodies—the spiral nebulae. When we have actually seen
that great arch swung over the equator of the planet without any visible
connexion, we cannot bring our minds to rest. We cannot simply admit that such
is the case, and describe it as one of the observed facts in nature, not
admitting or requiring explanation. We must either explain its motion on the
principles of mechanics, or admit that, in the Saturnian realms, there can be
motion regulated by laws which we are unable to explain.
The issue in Maxwell’s model (1856) is what would happen if a small planet collides with aother planet.
The initial debris can coalesce together and form a Moon if the new debris/moon
is outside a distance set by the Roche limit (1948), If it is within the Roche
limit the gravitational tidal forces will disintegrate any moon bring formed
under their own gravity. Inside the Roche
limit, the dispersed orbiting material
will form rings.
Maxwell’s
model (1856) had dust particles under mutual gravitational interaction moving
in closed orbits forced by Saturn’s gravitational field. The outer parts of
Saturn’s rings orbited at a slower speed than the inner parts. This was proved
by Doppler Shift measurements forty years later. The
ring is actually made of millions of tiny moons orbiting Saturn and obeying
Kepler’s third law (P2 proportional to a3, where P is the orbital period of the planet
and a is the semi-major axis of the
orbit). It is of interest to note that a poetic right-brain mind foresaw (1660)
the presence of million’s of moons in Saturn’s rings before Maxwell proved them
with his left-brain analytic mind. The poet was Jean Chapelain, a friend of
Christian Huygens who first proposed (1655) that there is a ring surrounding
Saturn after Galileo first observed (1610) first observed Saturn’s ring-like
feature.
Although
Maxwell did not look for an application of his model for Saturn’s rings his
concept was new. I learnt from Kragh1 that the idea of atoms being structured
like a planetary system was considered by Wilhelm Weber in the 1860-70s before
electrons as negatively charged particles were considered.Weber’s credentials
in electrical forces were solid by that time having unified electrostatics
(Coulomb’s force, 1785) with electrodynamics (Amperes force, 1826)) with
Faraday’s induction (1831) and having first considered the value, c, Weber, a close collaborator of Gauss,
was expelled earlier from the university of Gottingen by Ernest Augustus I, Duke of
Cumberland, along with Gauss and two Grimm brothers as part
of the seven Gottigen Professors who refused to sign an oath In carrying the King's patent into effect, Weber
proposed an electrical theory in which neutral
ether consisted of positive and negative particles orbiting each other. For the
atom, Weber had a massive negatively charged central atom with tiny positively
charged particles revolving around them.
Maxwell’s
model also perhaps prompted Jean Perrin in 1901 (Nobel laureate for Brownian
motion, 1926) in a popular talk to think of a Saturn-like
model in which atoms are “constituted” by “one or more” masses which are “very
strongly charged with positive electricity” as well as “by a multitude of corpuscles, in the manner
of small negative planets” with “the total negative charge exactly equivalent
to the total positive charge, in such a way that the atom is electrically
neutral. (from Kragh1) .
Planetary Models for Atoms
Planetary Models for Atoms
Nagaoka’s
model (Fig 4, left; these illustrations are taken from the internet and do not appear in the original publications) showed considerable insights into orbits of charged
particles. It is perhaps better to quote the model from his paper He first
describes his modification Maxwell’s model as consisting (from his abstract and
quoted several times in the net) “ ... of
a large number of particles of equal mass arranged in a circle at equal angular
intervals and repelling each other with forces inversely proportional to the
square of distance; at the centre of the circle, place a particle of large mass
attracting the other particles according to the same law of force. If these
repelling particles be revolving with nearly the same velocity about the
attracting centre, the system will generally remain stable, for small
disturbances, provided the attracting force be sufficiently great.” He then
adds his model: “The present case will evidently
be approximately realized if we replace these satellites by negative electrons
and the attracting centre by a positively charged particle...” Nagaoka
sought t make contact with experiments when he added in his abstract “Such an ideal atom will not
be contradictory to the results of recent experiments on kathode rays, radioactivity, and other
allied phenomena. Nagaoka
abandoned his model four years later when it was impressed upon him that a ring
of negative charges would be unstable due to “disruptive repulsion” of the
electrons. Unlike Maxwell, Nagaoka had no Roche limit to stimulate thought on
the stability of charges in a ring.
Thomson’s
model has Nagoka’s planar ingredients: “... a sphere of uniform positive
electrification, and inside this sphere a number of corpuscles arranged in a
series of parallel rings ... “. He also considers a three-dimensional
partitioning in which the rings are “...so
arranged that those which contain a large number of corpuscles are near the
surface of the sphere while those in which there are a smaller number of
corpuscles are more in the inside. ... they will arrange themselves in a series
of concentric shells ... .” Thomson also addressed himself to a
phenomenology that yields some of the characteristics of spectral lines thus: “... if all the corpuscles in one atom have
the same angular velocity, the frequency of the vibrations produced by the
rotation of the ring of corpuscles is proportional to the number of corpuscles
in the ring; and thus in the spectrum of each element in the series there would
be a series of frequencies bearing the same ratio to each other...”.
Thomson’s model for the corpuscles essentially involves their relative
positioning being static inside the ring.
Removing a
negatively charged electron would leave an opposite charge behind, so that for
the sake of neutrality one could consider each electron being bound to a
positive charge (Fig 4, second from right), This is Lenard’s model. Lenard
proposed that in an atom of a heavy
substance (he chose platinum) the “most impenetrable proper volume” of the atom
would have a size which is about one-thousandth of the actual atom ... while
the remainder is “as empty as the sky”. This empty space has “only fields of
force” as in free ether. Lenard then proposed the existence of “electrically
neutral” dynamids as “the centres of
their fields” possessing same amounts of
negative and positive electricity . The
negative electricity he identifies with that found in cathode rays while the
“positive electricity, on the other hand, appears to be something much more
specifically proper to the atoms of matter.”
In
his 1911 paper Rutherford gives credit to Geiger and Marsden. He has noted:
The observations, however, of Geiger and Marsden on the scattering of a rays indicate that some of the a particles must suffer a deflexion of more than a right angle at a
single encounter. They found, for example, that a small fraction of the
incident alpha particles, about 1 in 20,000, were turned through an average
angle of 90o in passing through a layer of gold-foil about
"00001/4 cm. thick, ... It seems reasonable to suppose that the deflexion
through a large angle is due to a single atomic encounter ... The theory of Sir
J. J. Thomson is based on the assumption that the scattering due to a single
atomic encounter is small, and the particular structure assumed for the atom
does not admit of a very large deflexion of an a particle in traversing a
single atom, unless it be supposed that the diameter of the sphere of positive
electricity is minute compared with the diameter or' the sphere of influence of
the atom.”
It
may be supposed that at the time Rutherford made his model, he must have been
immersed in the best traditions of science and he did not propose any further
than he could prove. He was therefore cautious to a fault (by present-day
standards) when he writes
the deductions from the theory are independent of whether the central
charge is supposed to be positive or negative. For convenience, the sign will
be assumed to be positive. The question of the stability of the atom proposed need
not be considered at this stage, for this will obviously depend upon the minute
structure of the atom, and on the motion of the constituent charged parts. ... Considering
the evidence as a whole, it seems simplest to suppose that the atom contains a
central charge distributed through a very small volume, and that the large
single deflexions are due to the central charge as a whole, and not to its
constituents. At the same time, the experimental evidence is not precise enough
to negative the possibility that a small fraction of the positive charge may be
carried by satellites extending some distance from the centre.
Rutherford gives
adequate credit to Nagaoka’s Saturnian model
when he noted “From
the point of view considered in this
paper, the chance of large deflexion would practically be unaltered, whether
the atom is considered to be a disk or a sphere.” He further modestly adds
... it has not so far been found possible to obtain definite evidence to
determine whether it (the central charge) be positive or negative. It may be possible
to settle the question of sign by consideration of the difference of the laws
of absorption of the b particle to be expected on the two hypotheses, for the effect of
radiation in reducing the velocity of the b particle should be far more marked with a positive than with a negative
centre
Rutherford’s 1911 paper spent considerable time on the experiments of Crowther with b rays that seemed to support Thomson;s model. In private correspondence with Bragg, Rutherford writes (fom Mansoor Niaz’s article, From Cathodes Rays to Alpha Particles to Quantum of Action...) “...I have looked into Crowther’s scattering paper carefully, and the more I examine it the more I marvel at the way he made it fit (or thought he made it fit) J. J.’s theory . . . I am quite sure the numbers of the earlier part of the curve [Crowther’s] were fudged.”
To be fair, most of Rutherford’s paper
was really reporting of the results of Geiger and Marsden. It does not look
like a bold paper with assertions of a dramatic picture. In a 2011 article in
the CERN courier Campbell writes that after his 7th May 2011 lecture
on the model of the atom, “... it created no great stir among scientists and the
public at the time. Three nights after his announcement, Rutherford addressed
the Society of Industrial Chemists on ‘Radium’. The nuclear atom was not
mentioned by Sir William Ramsay in his opening address to that year’s meeting
of the British Association ... Rutherford’s busy life continued as normal:
accepting a Corresponding Membership of the Munich Academy of Sciences; giving
talks on all manner of subjects but the nuclear atom --- motoring in the car
recently purchased with the money that had accompanied his Nobel prize ...”
Is there a Roche-like limit in the Saturn model for the atom?
Although the Saturnian model played such an important role in the planetary model for the atom, I have not seen any mention of the relevance of Roche-like limit for stabilizing an electron-ring, say, in Thomson's model. While perusing the internet I came across Fedele’s
article “From Maxwell's theory of Saturn's rings to the negative mass
instability” (Phil. Trans. R. Soc. A, vol. 366 pp 1717-1733
(2008)). I am not proficient at all in this area (as in many
others) but it does not deter me from trying even if it may look like a
cut-and-paste effort no matter how much I try. Fedele was writing about a mechanism by Nielsen and
co-workers that was put forward a hundred after Maxwell. . There is an analogy
between Saturn’s rings and the rings
of particle accelerators in which the particles are
under the action of electromagnetic forces (instead of the gravitational field
of the planet). In this
mechanism particles repel each other instead of attracting each other as in
Maxwell’s mechanism.
Let
us consider a relativistic charged particle beam travelling with velocity
close to that of light in a circular accelerator. The synchronous particle does
not change its energy or the radius of its circular orbit Because of repulsion between the particles one may think
that the beam would be unstable in a ring.
Bunching of charged particles can also take place for charged particles
undergoing circular motion in a particle accelerator. When particles are
accelerated towards the speed of light they increase their momentum by having a
larger radius of curvature. When a particle gains energy because of repulsion
it could its rotation rate. Particles leading the bunch in energy therefore
fall back into the bunch and the bunch is spontaneously retained. There is a
transition energy (akin perhaps to a Roche limit) above which normally
repelling particles begin attracting each other through what has been termed a
negative mass effect since the particles are drawn in the opposite
direction towards which the total force is acting, Maxwell’s
gravitational mechanism for the formation of Saturn’s rings is the
gravitational analogue for the bunching of charges.
One
wonders what Nagaoka/Thomson would have thought about their models if they knew
about the particle accelerators and (what I think) the Roche-limit-like transition energy.
One of my
most memorable technological events is the Voyager I mission which sent back
pictures from distant planets after travelling several years and millions of
miles. It has 1977 technology in it. Voyager I fires up my admiration like
nothing else can about the spirit of man as an inquisitive and accurate experimentalist.
It is now
at the edge of the solar system along a “magnetic highway” being powered by a
plutonium battery. It is still being monitored and it is still sending
data and still surprising scientists. . As its disrector for the last thirty
five years or so, Edward Stone, has said. “The new region
isn't what we expected, but we've come to expect the unexpected from Voyager.”The planetary community has learnt so much from Voyagers and the
subsequent missions. The legend of Voyager and its service to mankind must be
rememebered in some way; say, by the Nobel peace prize that will restore to it
some of the pride after the recent disastrous awards.
Among my
1980 memories are the pictures (Fig 5, from internet) sent by Voyagers about
Saturn’s outermost discrete F-ring. It is not important to record the chronology;
what I remember is the wave-like nature of the rings and it reminding me of de
Broglie waves for the atom’s rings. Later Cassini images (Fig 5 inset) and analyses
showed the role of the moons. A video
that is taken in 2005 which I like is at http://www.youtube.com/watch?v=fdUlpeUFfxI
. Prometheus and Pandora play an important role not only in holding together (shepherding)
these rings in their place but also in making these rings the “most active in
the Solar system”. Prometheus orbits Saturn faster than the material in the
F-ring. The attractive gravitational influence of Prometheus carves out new
periodic channels in the ring when it borrows some of the material. It then
gives it back. This exchange of mass gives it the equivalent of a mass
resonance”
I cannot
help imagining that if I take the outermost F-ring of Saturn as its “valence”
ring that is most sensitive to external influences, the quantum models for atoms
could have taken a different shape.
Models are
built by observations. The model lies in the experience of the observer. I am
reminded of another Thurber cartoon.